|

|
Volume 14, Number
11 - November 2014
Hello from Food Label News.
The news this Fall is all about preparing for the upcoming
changes to food labels. We help you get ready for your
re-labeling initiatives with a focus on servings per
container this month. There are several important
differences between the current regulations and the proposed
rule — so read on! Questions? Comments? We welcome your
dialogue in the Food Label Community. It's the virtual water
cooler to catch up and exchange
ideas.
This time of year we especially give thanks for our valued
clients and the ability to do the work we love.
|
|
Label Changes are Coming: Be Prepared
with
Part 3 of 4-part Series
The FDA proposed changes to nutrition labeling would affect
virtually every product label in the U.S. FDA's evaluation
period is underway and food labelers are readying their
products for major re-labeling initiatives.
|
|
|
In this issue, we highlight one of the
important differences between the current
regulations and the proposed rule: changes
to U.S. nutrition labeling for servings per
container. Here is a summary of key
points: |
|
• |
Packages that are 150% or less of the
Reference Amount Customarily Consumed (RACC)
will continue to be labeled as a
single-serve container.
|
|
• |
Packages that are 150% to 200% of RACC will
continue to be labeled as a single-serve container
when the RACC is less than
100g/100mL. |
|
• |
Currently, packages that are 150% to 200% of
RACC where the RACC is 100g/100mL or more
may be labeled as either 1 or 2 servings.
Under the proposed rule, these packages will
need to be labeled as a single-serve
container. |
|
• |
Under the proposed rule, most packages that
are 200% to 400% of RACC will need to be
labeled in a dual column format showing both
per serving and per container values. Some
exemptions apply, for example: 1) small
packages that use a tabular or linear
layout, 2) products that require further
preparation and voluntarily include "as
purchased" and "as prepared" values, 3) bulk
products used primarily as ingredients such
as flour, sweeteners or oils, 4)
multi-purpose bulk products such as eggs,
butter or margarine, 5) multi-purpose baking
mixes. |
|
• |
Packages that are more than 400% of RACC
will continue to be labeled as a multiple
serving container. |
|
See a
quick comparison of current
regulations vs. the proposed rule for U.S. nutrition labeling of
single-serve containers based on package size. |
|
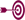 |
Those responsible for nutrition labeling will want to
fully understand how the proposed regulations will affect both the
Nutrition Facts panel and the claims made for the their products.
Thorough pre-planning for the new regulations will help you assess the
impact on your product labels.
Ask about our proposed rule label audits. |
|
|
What's News in the Food Label
Community
|
|
|
Reader Q&A
Find
answers to our readers'
questions or send us
your question for an
upcoming issue.
|
Q. |
Are manufacturers allowed to use the term "and/or" twice
or more in the ingredient statement but use only one of
those ingredients or a combination of any of those
ingredients separated by the term "and/or"? For example,
if a manufacturer lists "Calcium Caseinate and/or Milk
Protein Concentrate and/or Milk Protein Isolate" in the
ingredient statement of a protein shake, can they use
only one of the three ingredients or any combination of
ingredients to make this product?
−
J.C., California,
Manufacturer
|
|
A. |
FDA does not give permission to use "and/or" ingredient
labeling for calcium caseinate, milk protein
concentrate, or milk protein isolate. If all three are
listed in the ingredient statement, all three must be
present in the product.
Read more. |
|
|
|
|
|
|
|
What Matters in Food Labeling
Food Label News,
now in its 14th year, is a monthly e-newsletter reaching
over 8,000 subscribers around the world. We
welcome your colleagues to subscribe for news and insights
about food labels:
www.foodlabels.com/subscribe
|
|
|
|
|
Your Virtual
Food Label Partner
Food Consulting Company,
founded in 1993, provides nutrition analysis, food labeling,
and regulatory support for more than 1,500 clients worldwide.
Our
guarantee: 100% regulatory compliance.
Contact us
for the help you need now.
You may reprint all or part of this newsletter
provided you attribute it to Food Label News
and include a link to
www.foodlabels.com.
© 2014. Food Consulting Company, Del Mar, CA. All rights reserved.
|
|